
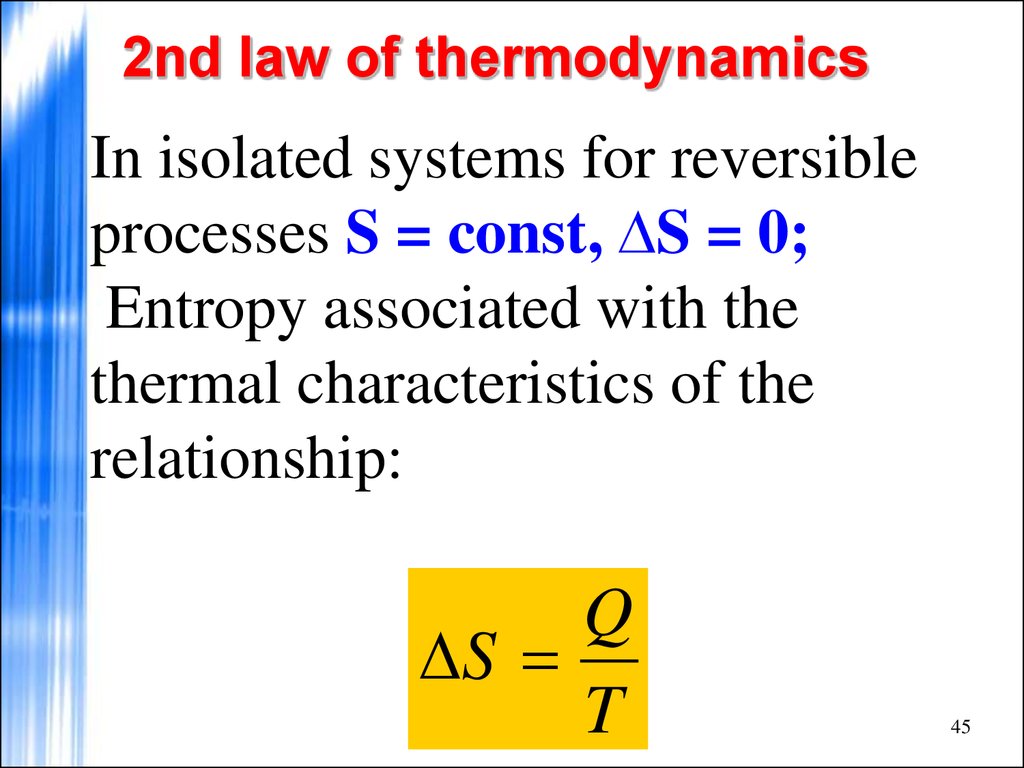
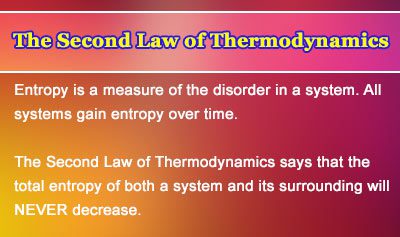
And “far from equilibrium” is the wild west to theoretical physicists and chemists: unpredictable and untidy. This simple, general equality provides precise information about many different types of thermodynamic systems at equilibrium.īut you and I and most of the world are far from equilibrium. (For example, our cake’s scent molecules reach equilibrium after they’ve fully filled the kitchen.) At equilibrium, the second law strengthens to an equality: $latex S_f = S_i$. That happens when large-scale properties - such as temperature and volume - remain constant, and no net flows of anything - such as energy or particles - enter or leave the system. The inequality is useful but vague, because it doesn’t tell us how much the entropy will grow, except in a special case: when the molecules are at equilibrium. We sum up this behavior in an inequality: $latex S_f \ge\ S_i$, where $latex S_i$ is the molecules’ initial entropy and $latex S_f$ their final entropy. Accordingly, the scent of sponge cake wafts across the entire kitchen and never recedes. The second law of thermodynamics decrees that the entropy of every closed, isolated system (such as our kitchen, assuming the windows and doors are shut) grows or remains constant. If the volume encompasses the whole kitchen (after the molecules have had time to travel farther), the entropy is exponentially larger. If the volume just encloses the cake (as it does when the cake is freshest), the entropy is relatively small. A physicist might wonder: In how many ways can these molecules be arranged throughout the volume of space they currently occupy? We call this number of arrangements the molecules’ entropy. Scent molecules carrying heat drift away from the cake.
To understand its second law, imagine a sponge cake, fresh from the oven, cooling on a countertop.

Thermodynamics - the study of energy - originated during the 1800s, as steam engines drove the Industrial Revolution.

Since the steam engine began modernizing the world, the second law of thermodynamics has reigned over physics, chemistry, engineering and biology.


 0 kommentar(er)
0 kommentar(er)
